 The simplest description of charcoal is the cold hard black remains left over after your campfire has gone out. Essentially all the water has been evaporated off (Pyrolysis), along with some volatile constituents, leaving behind the familiar crusty crumbly black chunks. Now charcoal can be made from the bones of animals, or coal, but for medicinal use it has historically come primarily from plant-based sources such as hardwood, bamboo, coconut, or peat. But, what is left after the fire goes out is, apart from a few trace minerals, pure carbon, just like the carbon atoms that make up the soft graphites in a “lead” pencil or the 345 carat diamonds in a golden crown. What makes the carbons different from each other is their distinct physical structures.
The simplest description of charcoal is the cold hard black remains left over after your campfire has gone out. Essentially all the water has been evaporated off (Pyrolysis), along with some volatile constituents, leaving behind the familiar crusty crumbly black chunks. Now charcoal can be made from the bones of animals, or coal, but for medicinal use it has historically come primarily from plant-based sources such as hardwood, bamboo, coconut, or peat. But, what is left after the fire goes out is, apart from a few trace minerals, pure carbon, just like the carbon atoms that make up the soft graphites in a “lead” pencil or the 345 carat diamonds in a golden crown. What makes the carbons different from each other is their distinct physical structures.
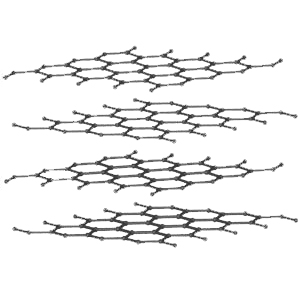 The carbon atoms in the graphite molecule lie in parallel sheets that allow them to “shed” easily in layers.
The carbon atoms in the graphite molecule lie in parallel sheets that allow them to “shed” easily in layers.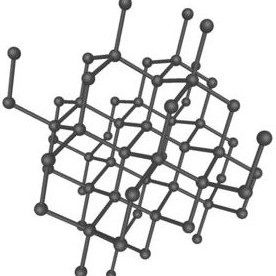 On the other hand the carbon atoms in a diamond are arranged in a 3-dimensional framework that reinforces each facet of its perfect cubic structure, making it the hardest mineral known.
On the other hand the carbon atoms in a diamond are arranged in a 3-dimensional framework that reinforces each facet of its perfect cubic structure, making it the hardest mineral known.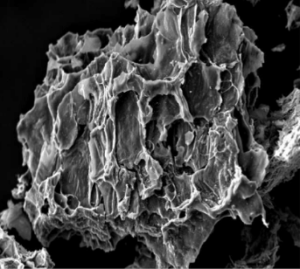 temperatures, the internal structure of the charcoal particle is further eroded creating an even greater surface area. Charcoal particles have a disproportionately high surface area as can be seen in this scanning electron micrograph of a particle of activated charcoal.
temperatures, the internal structure of the charcoal particle is further eroded creating an even greater surface area. Charcoal particles have a disproportionately high surface area as can be seen in this scanning electron micrograph of a particle of activated charcoal.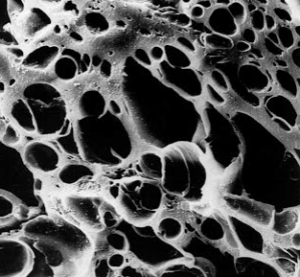 Charcoal manufacturers have developed different activation processes so that they can control to a large degree the size of these pores. In this way different charcoals can be designed to match the molecular sizes of the “pollutants” that are being targeted. These pores range in size from small to even smaller (50µ in diameter down to under 2µ).
Charcoal manufacturers have developed different activation processes so that they can control to a large degree the size of these pores. In this way different charcoals can be designed to match the molecular sizes of the “pollutants” that are being targeted. These pores range in size from small to even smaller (50µ in diameter down to under 2µ).  they detected the carbonmolecule C60 for the first time. It possessed unique physicochemical properties, extra stability, as well as some previously unexplained phenomena. To account for these features, they proposed a geodesic-like structure, one that essentially looks like the pattern on a soccer ball. Consequently the molecule was named after Buckminster Fuller, the inventor of geodesic domes (made famous at the 1967 World’s Fair). Buckminsterfullerene (fondly referred to as “Buckyballs” amongst some researchers) is the chosen name for C60, whereas the name fullerene is conveniently used for this whole family of closed carbon cages. They may not be as big as giant red stars, but these microscopic cells have gigantic appetites.
they detected the carbonmolecule C60 for the first time. It possessed unique physicochemical properties, extra stability, as well as some previously unexplained phenomena. To account for these features, they proposed a geodesic-like structure, one that essentially looks like the pattern on a soccer ball. Consequently the molecule was named after Buckminster Fuller, the inventor of geodesic domes (made famous at the 1967 World’s Fair). Buckminsterfullerene (fondly referred to as “Buckyballs” amongst some researchers) is the chosen name for C60, whereas the name fullerene is conveniently used for this whole family of closed carbon cages. They may not be as big as giant red stars, but these microscopic cells have gigantic appetites. In 1999 Eiji Osawa, and colleagues at the Toyohashi University of Technology in Japan, demonstrated that C60 can also be extracted from wood charcoal. As a result, many researchers now visualize charcoal as a structure made up of fragments of these “Buckyballs”. Along with the discovery of nanotubes or “Bucky onions” there is the suggestion of new magnetic and
In 1999 Eiji Osawa, and colleagues at the Toyohashi University of Technology in Japan, demonstrated that C60 can also be extracted from wood charcoal. As a result, many researchers now visualize charcoal as a structure made up of fragments of these “Buckyballs”. Along with the discovery of nanotubes or “Bucky onions” there is the suggestion of new magnetic and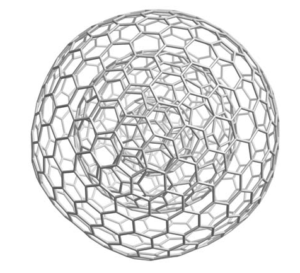 electrical properties. It all sounds a little bit like science fiction. No doubt in time these latest models for charcoal will again be modified. In the meantime charcoal still mystifies even the informed. Scientists marvel as they continue to ask, “How is charcoal able to…?”
electrical properties. It all sounds a little bit like science fiction. No doubt in time these latest models for charcoal will again be modified. In the meantime charcoal still mystifies even the informed. Scientists marvel as they continue to ask, “How is charcoal able to…?”
